1. Introduction
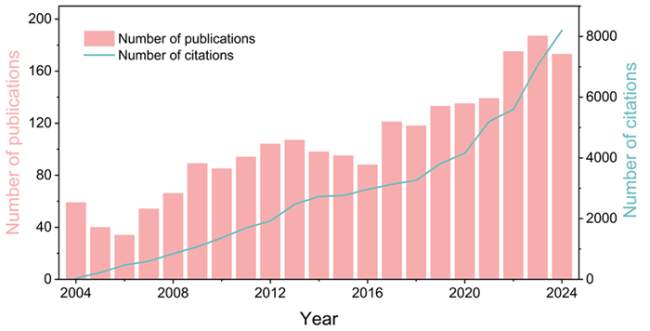
Fig. 1. Amount of publications and citations using “piezo*”, “ZnO or zinc oxide”, and “decompos* or remov* or degrad* or eliminat*” as keywords from 2004 to 2024 in Web of Science. |
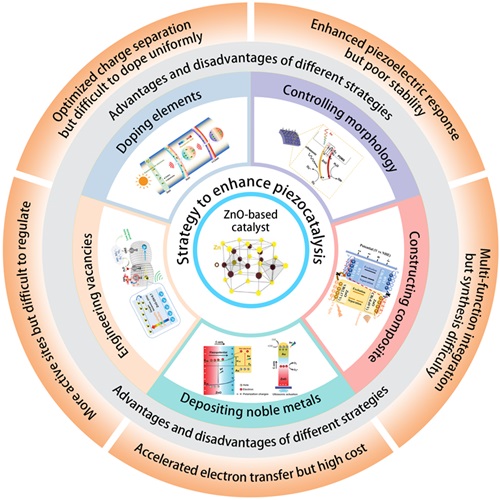
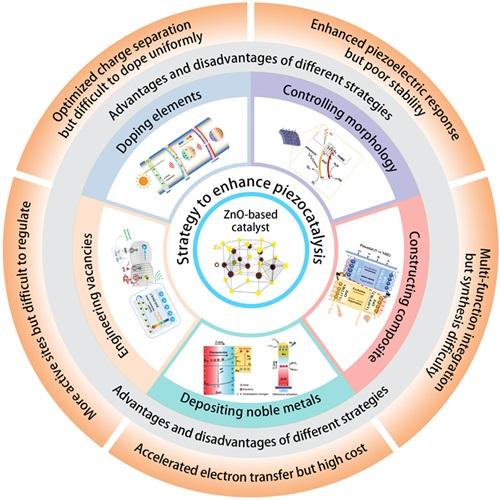
Fig. 2. Different strategies for enhancing the piezocatalytic performance of the ZnO-based catalysts. These inset figures are reproduced from ref. [75] (Copyright 2018, Materials Research Society); ref. [76] (Copyright 2019, Zhang et al.); ref. [77] (Copyright 2023, Elsevier Ltd.); ref. [78] (Copyright 2020, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim); ref. [54] (Copyright 2023, Wiley-VCH GmbH); ref. [79] (Copyright 2020, Elsevier); respectively. |
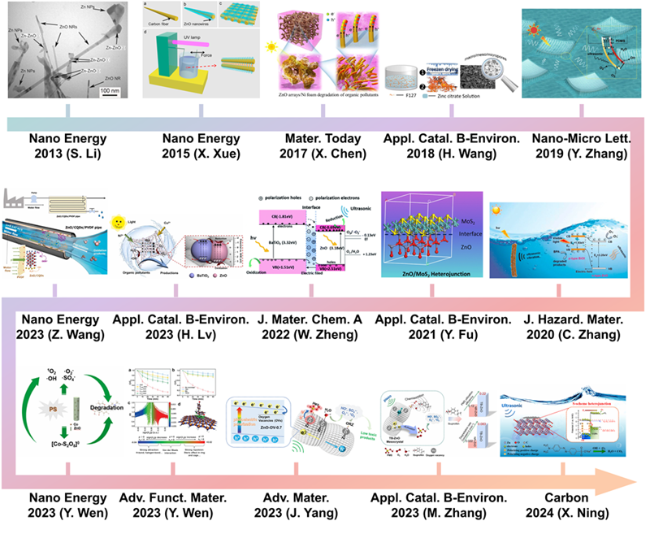
Fig. 3. Timeline for the typical strategies on enhancing piezocatalysis performance of ZnO-based catalysts for aquatic pollutants degradation. These inset figures are reproduced from ref. [80], [81] and [82] (Copyright 2013, 2015 and 2017, Elsevier Ltd.); ref. [83] (Copyright 2017, Elsevier B.V.); ref. [76] (Copyright 2019, Zhang et al.); ref. [84] and [85] (Copyright 2020, Elsevier B.V.); ref. [86] (Copyright 2022, The Royal Society of Chemistry); ref. [87] (Copyright 2023, Elsevier B.V.); ref. [88] and [89] (Copyright 2022 and 2023, Elsevier Ltd.); ref. [90] and [54] (Copyright 2023, Wiley-VCH GmbH); ref. [91] (Copyright 2023, Elsevier B.V.); ref. [92] (Copyright 2023, Elsevier Ltd.); respectively. |
2. Review necessity
3. Synthesis of ZnO
4. The strategy to enhance piezocatalysis of ZnO materials
4.1 Design principles
4.2 Enhanced piezocatalysis through controlling morphology of ZnO materials
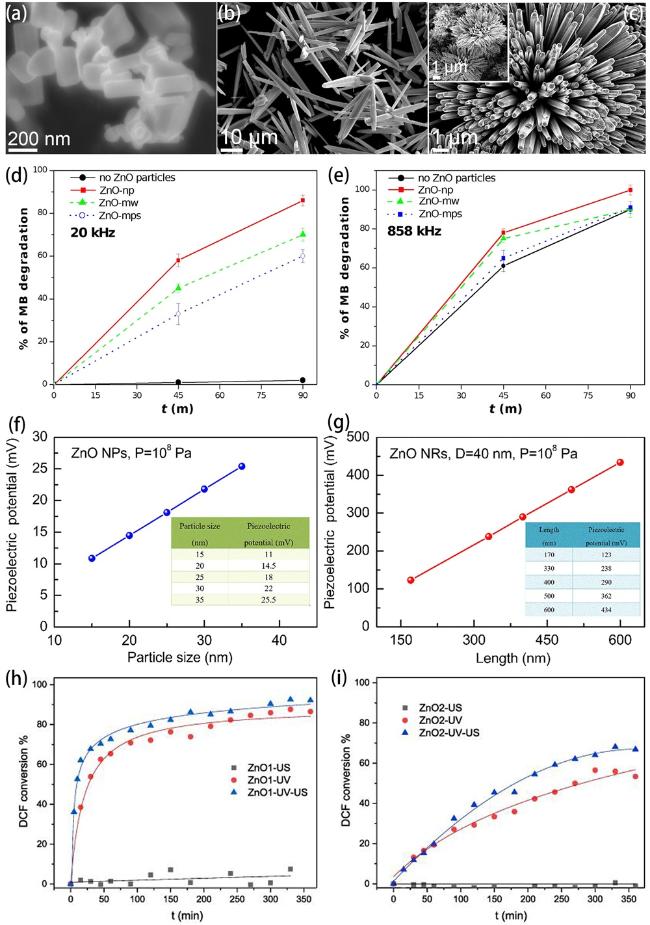
Fig. 4. (a-c) Microscopic (FESEM) images of ZnO nanoparticles (in particular nanorods), 1D microwires and multipods-like microparticles. MB degradation at 20 kHz (d) and 858 kHz (e) ultrasound in the presence of ZnO nanoparticles (ZnO-np), 1D microwires (ZnO-mw) and multipods-like microparticles (ZnO-mps) and without ZnO particles. Reproduced from ref. [145] (Copyright 2023, Troia et al.). (f) Piezoelectric potential curve versus particle size in granular nano-ZnO (ZnO NPs) under the fixed pressure of 108 Pa by COMSOL simulation. (g) Piezoelectric potential curve versus length in rod-like nano-ZnO (ZnO NRs) under the fixed diameter of 40 nm and cavitation pressure of 108 Pa by COMSOL simulation. Reproduced from ref. [146] (Copyright 2020, Elsevier Inc.). DCF conversion degree using ZnO1, i.e. ZnO nanorods (h) and ZnO2, i.e. star-shaped clusters (i) under ultrasound (US), ultraviolet light (UV) and both of them (UV-US). Reproduced from ref. [147] (Copyright 2021, Meroni et al.). |
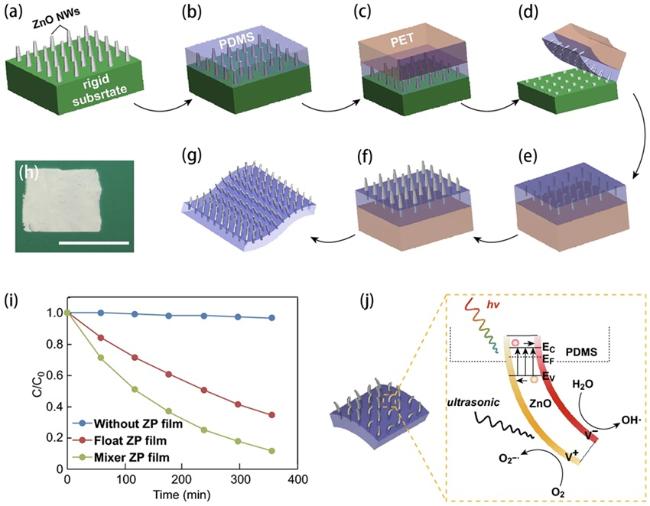
Fig. 5. (a-g) Schematic illustration of the two-step fabrication process of the vertically aligned ZnO NW array on a polymer matrix. (h) Picture of the as-prepared ZP film (1 × 1 in.2). Scale bar: 1 in. (i) Degradation curves of the MB dye in the presence of an ultrasonic field and UV light irradiation. (j) Schematic diagram of the piezo-photocatalytic mechanism. Reproduced from ref. [76] (Copyright 2019, Zhang et al.). |
4.3 Enhanced piezocatalysis through constructing composite
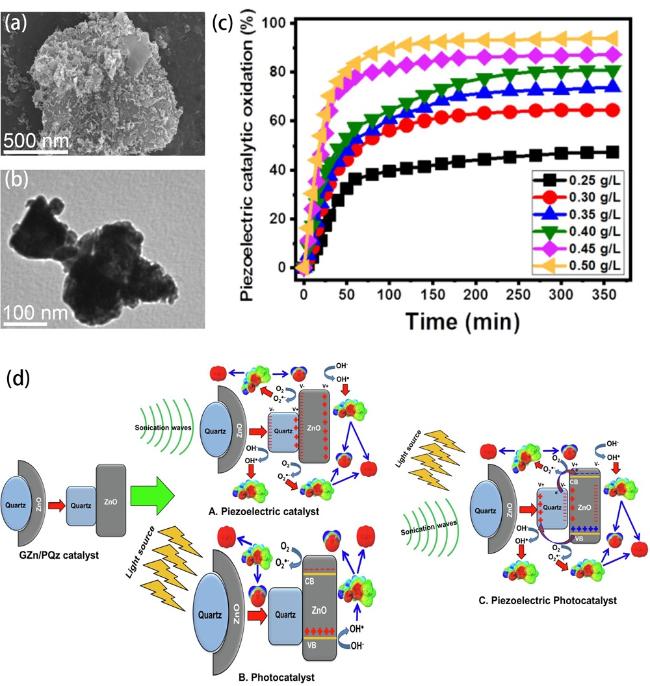
Fig. 6. (a) SEM image and (b) HRTEM image of the GZn/PQz composite. (c) The oxidation of IBF by piezoelectric catalysis with different doses of GZn/PQz catalyst. (d) General mechanism of IBF oxidation over GZn/PQz as piezoelectric catalyst, photocatalyst, and piezo-photocatalyst. Reproduced from ref. [153] (Copyright 2021, Elsevier B.V.) |
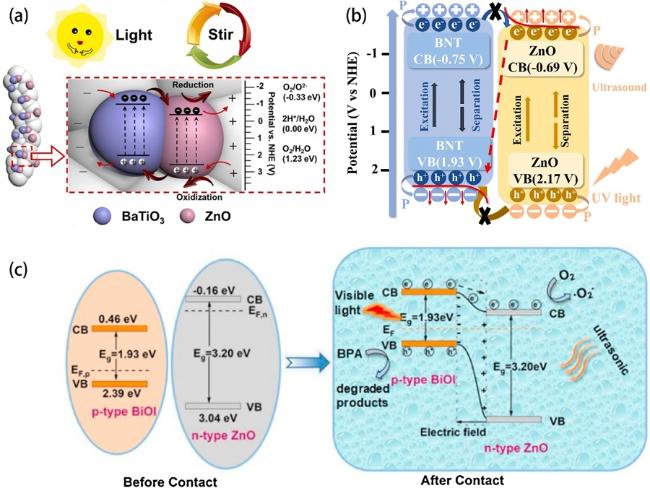
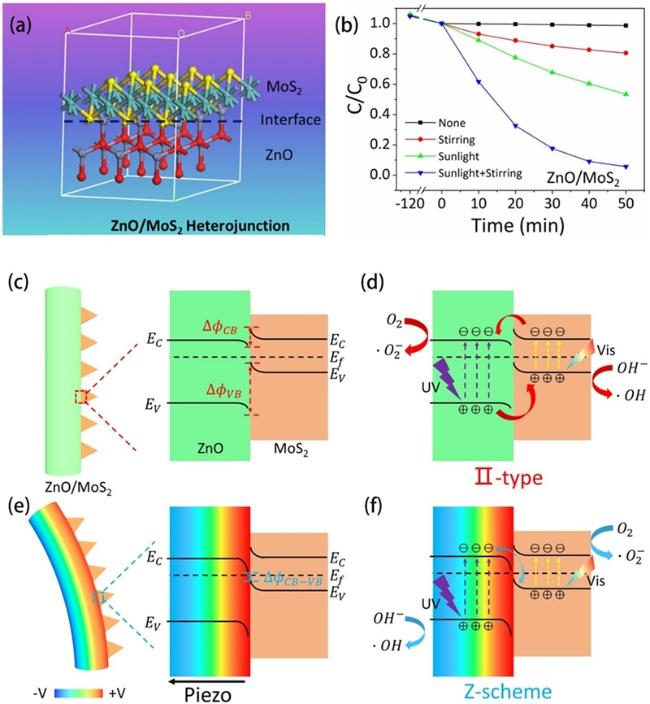
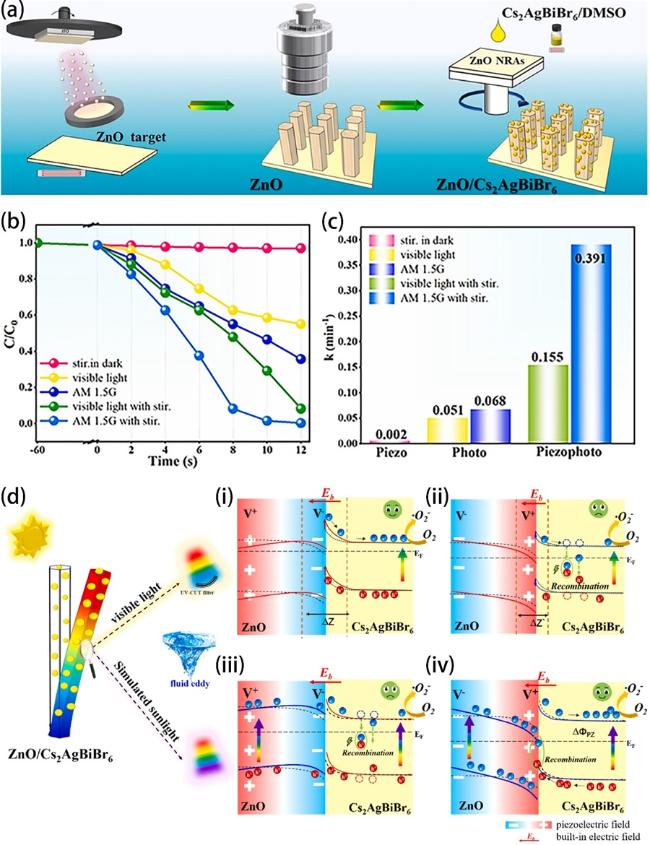
Fig. 7a, it’s a typical type II heterojunction. There is a partly overlapping band gap between BaTiO3 and ZnO, and the electrons in BaTiO3 will migrate and accumulate on the conduction band (CB) of ZnO, while the holes from ZnO transfer to the valence band (VB) of BaTiO3 under this circumstance. The procedure can be improved by a built-in electric field resulting from piezoelectric polarization under stir, consequently hinders the reassociation of electrons and holes obviously in BaTiO3 and ZnO and more charge carriers react with O2 and H2O to generate free radicals to enhance degradation efficiency. Zhou et al. also revealed that ZnO/BaTiO3 heterostructures have an enhanced piezophototronic effect [173]. The oxidation reaction rate constant of dyeing wastewater degradation with ZnO/BaTiO3 can be up to 1.20×10-1 min-1 under concurrent ultrasound and simulated sunlight. There are else materials such as CdS [165] and (Na0.5Bi0.5)0.94Ba0.06TiO3 [174] combined with ZnO to construct type II heterojunctions for greater piezocatalysis/piezo-photocatalysis activity. Different from type II heterojunction, Fig. 7b demonstrates a Bi0.5Na0.5TiO3/ZnO (BNT/ZnO) Z-scheme heterojunction for piezo-photocatalytic water remediation [77]. The BNT/ZnO Z-scheme heterojunction reserves electrons and holes which own more powerful redox capacity due to the recombination of electrons in the CB of ZnO and holes in the VB of BNT. Fu et al. synthesized a ZnO/MoS2 heterojunction (Fig. 8a) on Ni foam [85]. As we can see in Fig. 8c-f, the ZnO/MoS2 II-type heterojunction can be converted to direct Z-scheme heterojunction by flowing-induced piezoelectric field for enhanced sunlight photocatalytic performances. Under stirring-sunlight irradiation for 50 min, the degradation efficiency of methyl orange reaches up to 92.7%, versus 50.6% under sole sunlight irradiation (Fig. 8b). Nevertheless, 50% of the charge carriers recombine at the interface theoretically in Z-scheme heterojunctions, which decreases the energy conversion efficiency [175]. In 2016, Liu et al. achieved boosted piezoelectric and piezo-phototronic performance based on p-CuI/n-ZnO heterojunction [176]. Over the past few years, many works focused on the fabrication of ZnO-based p-n heterojunction for high piezo-photocatalytic efficiency [167,177-179]. But what is p-n heterojunction? When a p-type semiconductor and a n-type semiconductor are in contact, they form a p-n junction with a space-charge region at the interfaces, and thus produce a built-in electrical potential that can move the electrons and holes in the opposite direction [180]. Zhang et al. prepared a p-n heterojunction of BiOI/ZnO (Fig. 7c) and this BiOI/ZnO nanorod arrays degraded the bisphenol solution completely within 30 min under concurrent visible-light irradiation and ultrasonic vibration [84]. Such prominent catalytic activity can be ascribed to the synergy of the piezoelectric field created by ZnO nanorod and the built-in electrical potential in the p-n heterojunction. Recently, S-scheme heterojunctions garnered interest in the domain of ZnO-based piezo-photocatalyst because of its strong redox potential, which can be attributed to the internal electric field, band bending, and Coulombic attraction [181]. As shown in Fig. 9a, Lv et al. constructed a S-scheme heterojunction of ZnO/Cs2AgBiBr6 nanorod array (ZC NRA) via a seed-assisted hydrothermal method followed with a multi-step spin-coating process [163]. Under simulated sunlight irradiation with magnetic stirring, the RhB is almost degraded absolutely by the fabricated catalysts in 12 min and the degradation kinetic constant is 0.391 (Fig. 9b and c). The dramatical degradation performance of the ZC NRA can be ascribed to the coupling mechanism of the S-scheme heterojunction and the piezoelectric field, which contributes to the efficient separation of charge carriers (Fig. 9d iv).
Table 1. Catalytic performance of different ZnO-based composites toward pollutant degradation. |
| Materials | Model pollutants | Degradation efficiency | External conditions | Pros and cons | Ref. |
|---|---|---|---|---|---|
| ZnO/piezoelectric quartz core-shell | IBF (50 mg L-1) | 100% for 40 min | Ultrasound (20 kHz) + metal halide lamp (400 W) | Green synthesis/ many steps | [153] |
| MoOx/ZnS/ZnO ternary complex | RhB (10 mg L-1) | ~ 99% for 90 min | Ultrasound (40 kHz, 120 W) | Good performance/ environmentally unfriendly | [154] |
| ZnO NR/PVDF-HFP spongy film | MO (5 mg L-1) | 95% for 75 min | Stirring (1000 rpm) + mercury-xenon lamp irradiation. | Easy to recycle/ environmentally unfriendly, many steps | [158] |
| Ultrathin ZnO/Al2O3 | MO (50 mg L-1) | 100% for 15 min | Ultrasound (~40 kHz, 100 W) | Excellent performance/ environmentally unfriendly, many steps | [160] |
| ZnO@ZIF-8 core-shell | TC (50 mg L-1) | 91.5% for 40 min | Ultrasound (35 kHz, 180 W) | Good performance/ poor stability | [161] |
| ZnO@PVDF film | RhB (12 mg L-1) | ~ 97% for 100 min | Stirring + Xe lamp (300 W) | Bi-piezoelectric effect, more (100) polar plane exposure / environmentally unfriendly | [162] |
| BaTiO3/ZnO continuous nanofiber | RhB (5 mg L-1) | 98.94% for 90 min | Ultrasound (120 W) + Hg lamp (300 W) | Bi-piezoelectric effect/ environmentally unfriendly, many steps | [86] |
| ZnO/MoS2 nanoarray | MO (10 mg L-1) | 92.7% for 50 min | Stirring + Xe lamp (300 W) | Flowing-induced piezoelectric field/poor stability, many steps | [85] |
| BaTiO3//ZnO Janus nanofibers membrane | TC | 97.65% for 60 min | Stirring (800 rpm) + Xe lamp (300 W) | Simultaneously removal of multi-pollutants/ environmentally unfriendly, many steps | [87] |
| BiOI/ZnO nanorod arrays | BPA (10 mg L-1) | 100% for 30 min | Ultrasound (40 kHz, 90 W) + Xe lamp (300 W) | Expanded light absorption range, excellent performance/ poor stability, many steps | [84] |
| ZnO/Cs2AgBiBr6 nanorod arrays | RhB (10 mg L-1) | ~ 100% for 12 min | Stirring (500 rpm) + Xe lamp (300 W, equipped with AM 1.5G filter) | Excellent performance /environmentally unfriendly, many steps | [163] |
| GQDs/ZnO | MO | 96.1% for 60 min | Ultrasound (40 kHz, 150 W) | Excellent carrier separation/ many steps | [92] |
| Bi2WO6/g-C3N4/ZnO | RhB (5 mg L-1) | 95.1% for 20 min | Ultrasound (40 kHz, 80 W) | Expanded the charge transfer path/ environmentally unfriendly, many steps | [164] |
| CdS/ZnO | RhB (10 mg L-1) | 98.8% for 90 min | Ultrasound (40 kHz, 120 W) | Improved charge separation/ toxicity | [165] |
| ZnO/g-C3N4 nanoarrays | MB (10 mg L-1) | 93.70% for 120 min | Stirring (1000 rpm) + Xe lamp (300 W) | Flowing-induced piezoelectric field /many steps | [166] |
| CuS/ZnO nanowires | MB (5 mg L-1) | ~ 100% for 20 min | Ultrasound (200 W) + Xe lamp (500 W) | Excellent performance, easy to recycle/ many steps | [167] |
| ZnO/ZnS nanotube | MB (10 mg L-1) | 63.3% for 50 min | Ultrasound (120 W) + Hg-lamp (500 W) | Suppressed carriers recombination /low degradation rate constant | [168] |
| KNbO3/ZnO nanocomposite | MO (10 mg L-1) | ~ 100% for 90 min | Ultrasound (40 kHz, 120 W) + Xe lamp (300 W) | Improved charge separation/ many steps | [169] |
| g-C3N4[U]/ZnO | RhB (10 mg L-1) | 99% for 120 min | Ultrasound (40 kHz, 60 W) + visible light (50 W) | RhB degradation and H2 production/ many steps | [170] |
| ZnO/CuS | TC (30 mg L-1) | 85.28% for 60 min | Ultrasound (120 W) + Xe lamp (300 W, λ > 400 nm) | Narrow bandgap/ environmentally unfriendly, many steps | [171] |
| ZnO/SnS | Cr(VI) (20 mg L-1) | 98% for 35 min | Ultrasound | Cr(VI) removal/ environmentally unfriendly, many steps | [172] |
Fig. 7. (a) Piezo-photocatalytic mechanism of 2% BTO//ZO JNM under stir + light irradiation. Reproduced from ref. [87] (Copyright 2023, Elsevier B.V.). (b) mechanism schematic of RhB degradation through piezo-photocatalysis of BNT-1%ZnO. Reproduced from ref. [77] (Copyright 2023, Elsevier Ltd.). (c) Formation of p-n heterojunction between BiOI and ZnO, and probable separation process of charge carriers in the BiOI/ZnO nanorods under light and ultrasonic vibration. Reproduced from ref. [84] (Copyright 2020, Elsevier B.V.). |
Fig. 8. (a) Schematic illustration of lattice structure of ZnO/MoS2 heterojunction. (b) MO degradation efficiencies under different conditions. Energy band diagrams of ZnO/MoS2 without stirring (c) and under stirring (e). The migration of photogenerated charge carries and production of reactive oxygen species under sole sunlight irradiation (d) and stirring-sunlight irradiation (f). Reproduced from ref. [85] (Copyright 2020, Elsevier B.V.). |
Fig. 9. (a) Illustration of the synthesis process of the ZnO/Cs2AgBiBr6 nanorod array. (b) Degradation efficiencies of RhB using ZC0.5 under different situations (visible light, simulated sunlight, visible light with stirring, simulated sunlight with stirring) and (c) corresponding calculated k values. (d) Proposed mechanism of piezoelectric field and photosource-modulated photocatalysis of ZnO/Cs2AgBiBr6 S-scheme heterojunction, (i, ii) ZnO under visible light irradiation (λ > 420 nm) and (iii, iv) ZnO under simulated sunlight irradiation in ZnO/Cs2AgBiBr6 S-scheme heterojunction under opposite piezoelectric field directions. Reproduced from ref. [163] (Copyright 2023, Elsevier Ltd.). |
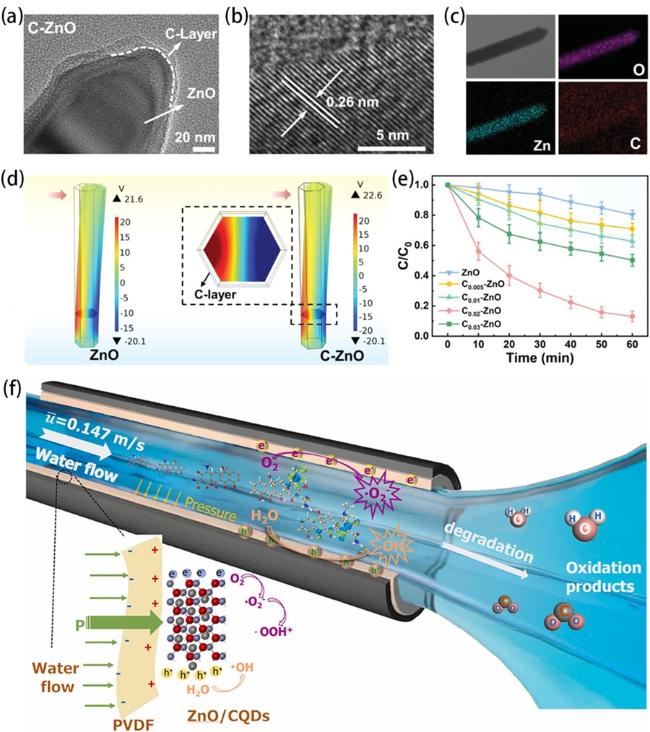
Fig. 10. (a, b) TEM images of C-ZnO nanorods. (c) Element mapping images of C-ZnO nanorods. (d) The results of piezoelectric potential distribution in ZnO and C-ZnO simulated by the finite element method. (e) The degradation of BPA over C-ZnO. Reproduced from ref. [90] (Copyright 2023, Wiley-VCH GmbH). (f) Mechanism of TC degradation with ZnO/CQDs/PVDF pipe piezoelectric catalytic system. Reproduced from ref. [88] (Copyright 2022, Elsevier Ltd.). |
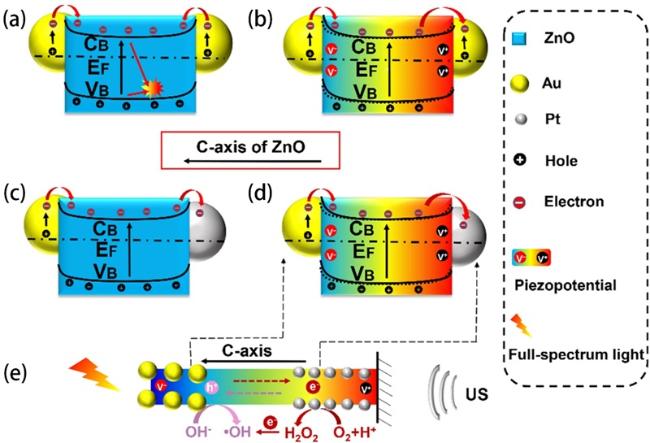
4.4 Enhanced piezocatalysis through depositing noble metals on the surface of ZnO materials
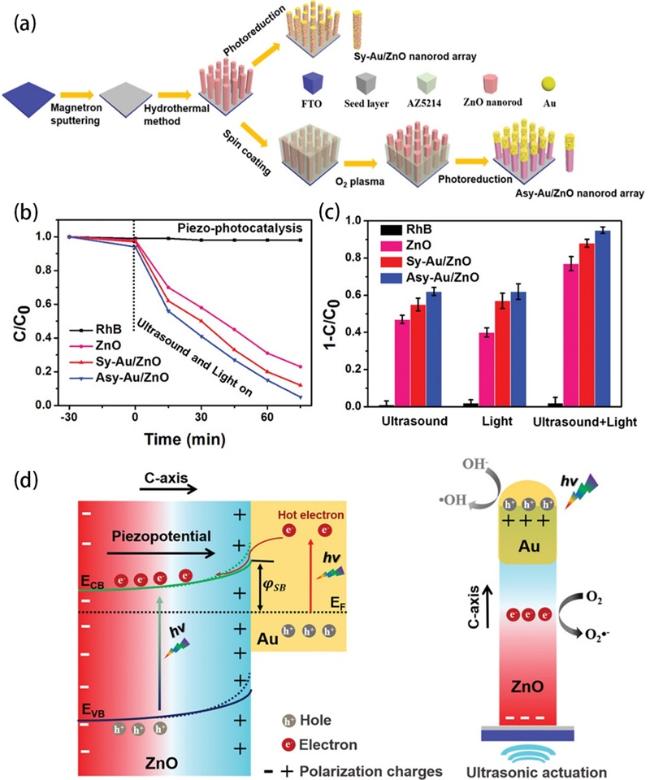
(Fig. 11a) to optimize piezo-photoelectric catalysis [78]. As illustrated in Fig. 11b and c, The Asy-Au-ZnO achieved a 95% decomposition of RhB in 75 min under all-spectrum light irradiation and ultrasonic actuation, higher than that of ZnO (77%) and symmetric Au-ZnO (88%). The greatly enhanced catalytic performance of the Asy-Au-ZnO was attributed to the unique 1D asymmetric architecture, which not only integrated the piezotronic effect of ZnO and plasmonic effect of Au nanoparticles, but also realized the spatially directed separation and migration of carriers (Fig. 11d). Zhang et al. further pushed the investigation about noble metal more deeply to dual noble metals [185]. With the help of Au nanoparticles and Pt nanoparticles, excellent carriers separation effect inspired the Au/ZnO/Pt system to boost the surface catalytic reaction for highly efficient dye degradation (Fig. 12d and e). In addition, coating noble metal on ZnO NRs which grow around piezoelectric PVDF substrate also realized outstanding piezo-photocatalytic activity [186].
4.5 Enhanced piezocatalysis through engineering vacancies
4.6 Enhanced piezocatalysis through doping elements into ZnO materials
Fig. 11. (a) Schematic of the fabrication process of the asymmetric Au-ZnO and the symmetric Au-ZnO nanorod array. (b) Piezo-photocatalytic degradation of RhB with different catalysts, and control sample without catalysts. (c) Summarized histogram of the RhB degradation under different conditions for 75 min. (d) Mechanism of the enhanced catalytic activity induced by piezotronic effect and unique asymmetric nanostructure under light illumination and ultrasound stimulation (, Schottky Barrier; and, the conduction band energy and valence band energy of ZnO, respectively;, energy of the Fermi level). Reproduced from ref. [78] (Copyright 2020, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim). |
Fig. 12. Mechanism of the enhanced catalytic performance via piezo-phototronic effect and the unique structure under ultrasonic wave and light irradiation (CB and VB, the conduction band and valence band of ZnO, respectively; EF, energy of the Fermi level). (a) AZA under light irradiation. (b) AZA under concurrent ultrasonic wave and light irradiation. (c) AZP under light irradiation. (d) and (e) AZP under concurrent ultrasonic wave and light irradiation. AZA, Au/ZnO/Au; AZP, Au/ZnO/Pt. Reproduced from ref. [185] (Copyright 2022, Elsevier Ltd.). |
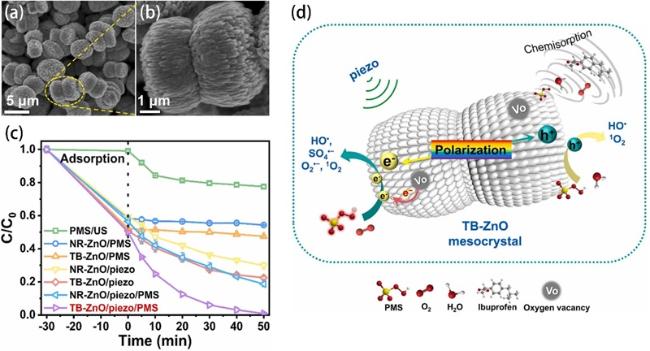
Fig. 13. (a, b) SEM images of TB-ZnO. (c) Degradation efficiencies of IBP in different catalytic systems. (d) IBP degradation mechanism with the TB-ZnO/piezo/PMS system. Reproduced from ref. [91] (Copyright 2023, Elsevier B.V.). |
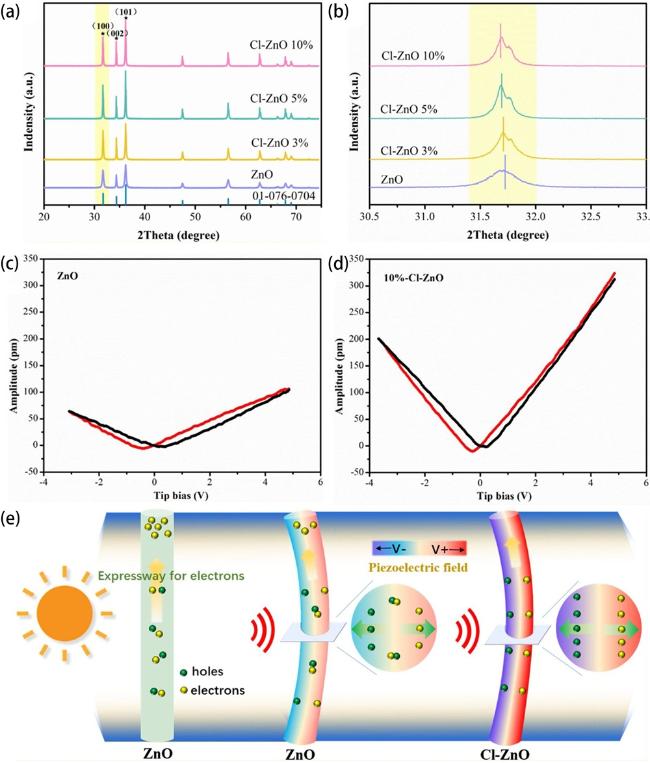
Fig. 14. (a) XRD patterns of undoped ZnO, 3%-Cl-ZnO, 5%-Cl-ZnO, 10%-Cl-ZnO and (b) an magnifying view of the ZnO (100) peak. The amplitude butterfly loop for (c) ZnO and (d) 10%-Cl-ZnO coated onto an indium tin oxide under ±10 V DC bias field along radial direction. (e) Schematic diagram for the catalytic mechanism of the ZnO and Cl-ZnO under different external conditions. Reproduced from ref. [79] (Copyright 2020, Elsevier). |
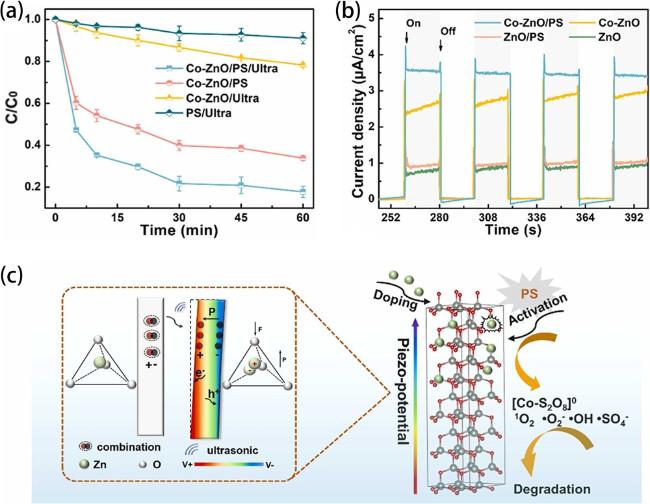
Fig. 15. (a) degradation curves of LVX under different conditions. (b) Transient piezoelectric current properties of ZnO and Co-ZnO with different systems. (c) Schematic diagram of piezo-promoted PS activation. Reproduced from ref. [89] (Copyright 2023, Elsevier Ltd.). |
4.7 Promising and advanced modification strategies
5. Mechanism analysis
5.1 Theoretical calculations
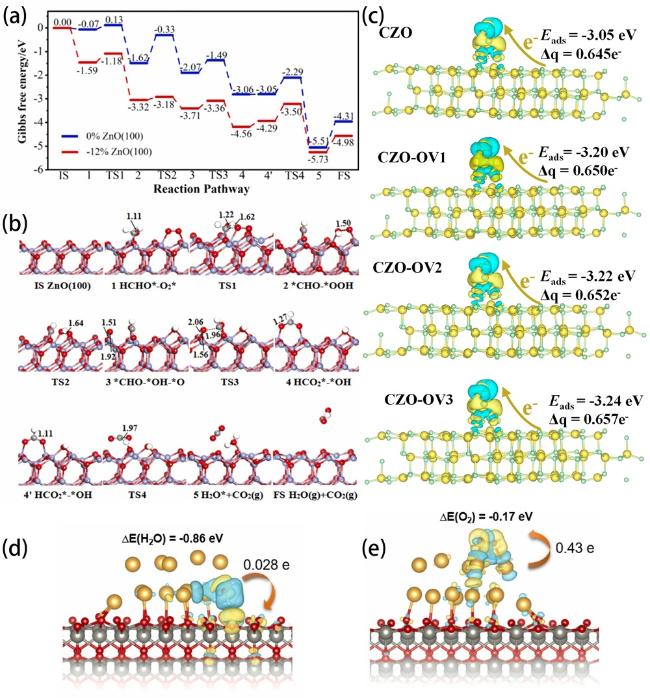
(Fig. 16d and e). The avoidance of competition at reaction sites can improve the reaction efficiency of hydrogen peroxide production through dual channels.
5.2 In situ characterization
Fig. 16. (a) Profiles of free energy for catalytic oxidation of formaldehyde onto ZnO (100) surfaces. (b) The computed transitional states and intermediate state structures for catalytic oxidation of formaldehyde onto -12% ZnO (100) surface. Reproduced from ref. [214] (Copyright 2024, Elsevier B.V.). Where -12% indicates the degree of compressive strain. (c) The charge density difference of the O2 molecule on cobalt-loaded ZnO with varying oxygen vacancy concentrations (the isosurface is adjusted to 0.0005 eV; the blue electronic cloud indicates electron gain and the yellow electronic cloud indicates electron loss). Reproduced from ref. [215] (Copyright 2024, Ran et al.). (d) H2O adsorption energy on ZnO sites of Au-ZnO model. (e) O2 adsorption energy on Au sites of Au-ZnO model. Reproduced from ref. [216] (Copyright 2024, Elsevier Ltd.). |
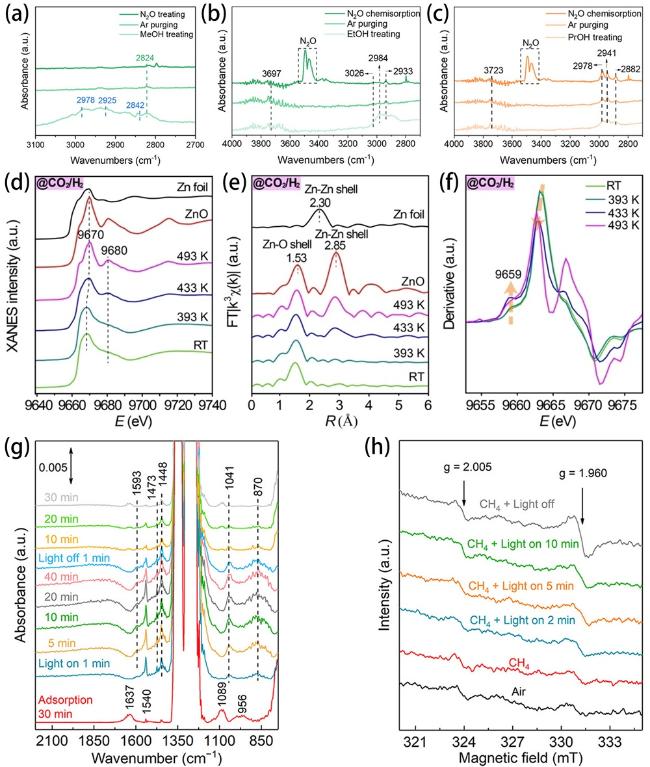
Fig. 17. In situ DRIFTS of Cu/ZnO in (a) H2/MeOH/H2O, Ar, and N2O atmospheres, (b) H2/EtOH/H2O, Ar, and N2O atmospheres, (c) H2/PrOH/H2O, Ar, and N2O atmospheres. Reproduced from ref. [226] (Copyright 2023, Wiley-VCH GmbH). (d) Normalized XANES spectra and (e) Fourier transforms of EXAFS spectra of 1ZnO/Cu(OH)2 (“1” denotes the number of ZnO atomic layer deposition cycles) during CO2 hydrogenation under a pressure of 0.8 MPa at different temperatures. (f)First derivative of the Zn K edge spectra of the 1ZnO/Cu(OH)2 inverse catalyst during CO2 hydrogenation at different temperatures. Reproduced from ref. [227] (Copyright 2022, Wiley-VCH GmbH). (g) In situ DRIFTS spectra for photocatalytic conversion of CH4 over ZnO-AuPd2.7%. (h) In situ EPR signals of ZnO-AuPd2.7% collected under different environments. Reproduced from ref. [228] (Copyright 2020, American Chemical Society). |
6. Summary and perspective
Fig. 18. Challenges and prospects of ZnO-based piezoelectric materials. |