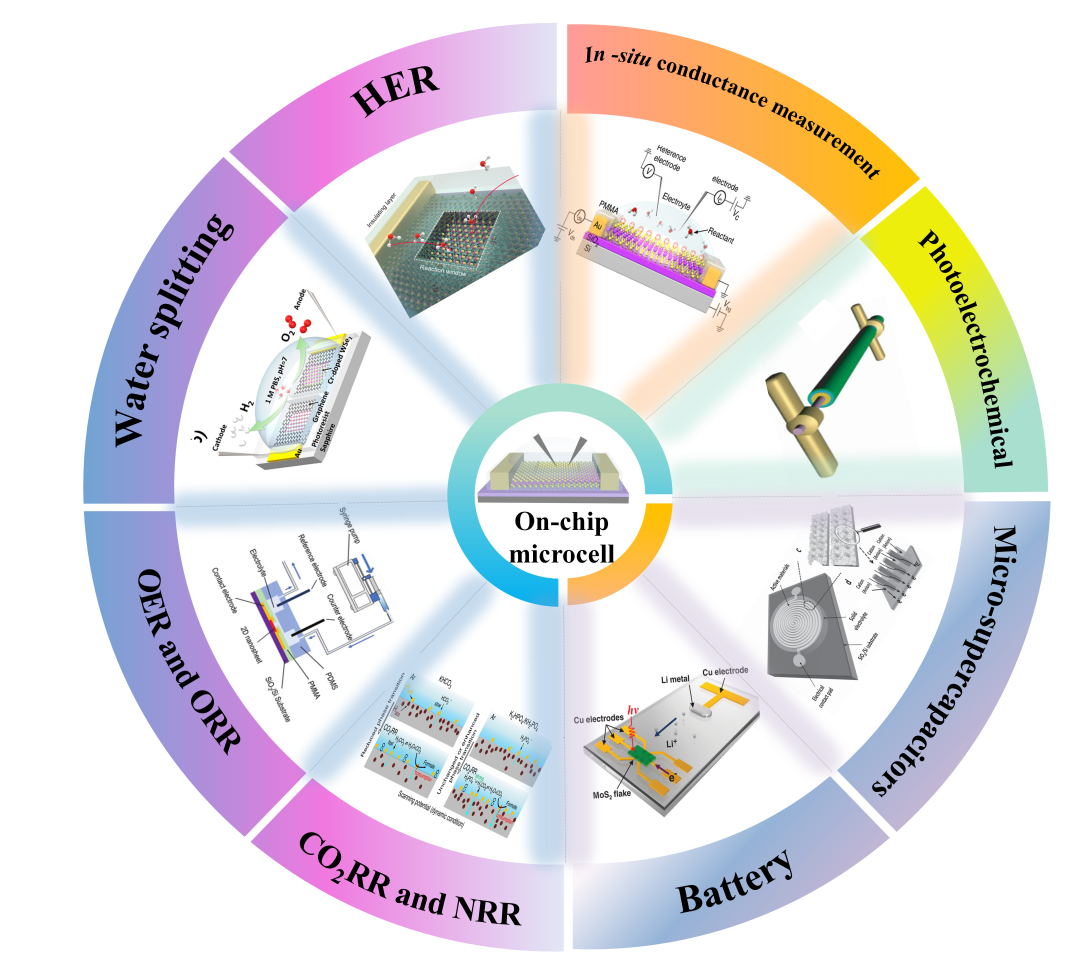
1 Introduction
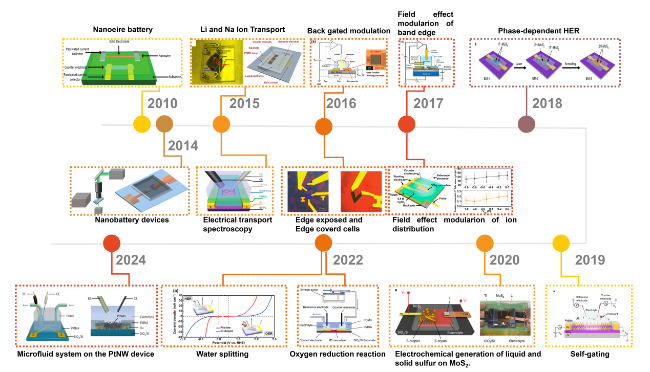
Figure. 1 Timeline for the on-chip microcell in electrocatalysis.[77] Copyright (2010) American Chemical Society; [78] Copyright (2014) Nature Publishing Group; [68, 79] Copyright (2015) American Chemical Society and Nature Publishing Group; [66, 80] Copyright (2016) American Chemical Society and Nature Publishing Group; [67, 81] Copyright (2017) American Chemical Society; [82] Copyright (2018) Nature Publishing Group; [69] Copyright (2019) Nature Publishing Group; [83] Copyright (2020) Nature Publishing Group; [84, 85] Copyright (2022) Wiley-VCH Verlag GmbH & Co and American Chemical Society; [86] Copyright (2024) Nature Publishing Group. |
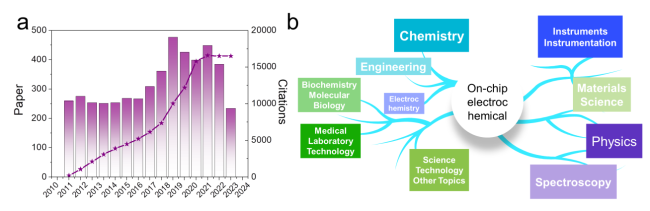
Figure. 2 (a) Annual number of publications by using keyword of “on-chip electrocatalysis” from Web of Science on January 7, 2025. (b) The hot spot in the total circulation. |
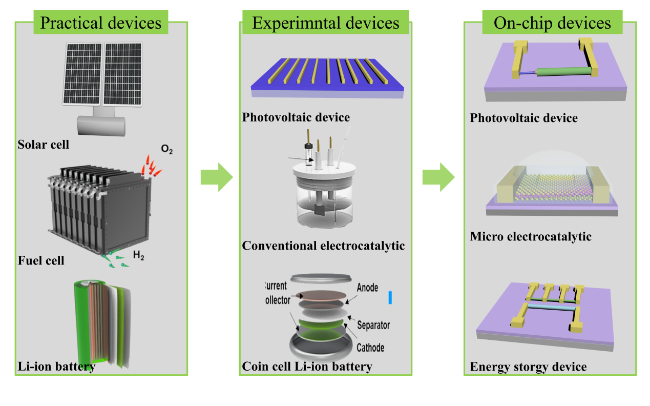
2 The preparation of on-chip devices
2.1 Photovoltalic devices
2.2 Energy conversion devices
2.3 Energy storage devices
3 Energy conversion
3.1 Hydrogen evolution reaction (HER) device
3.1.1 Identification of active sites
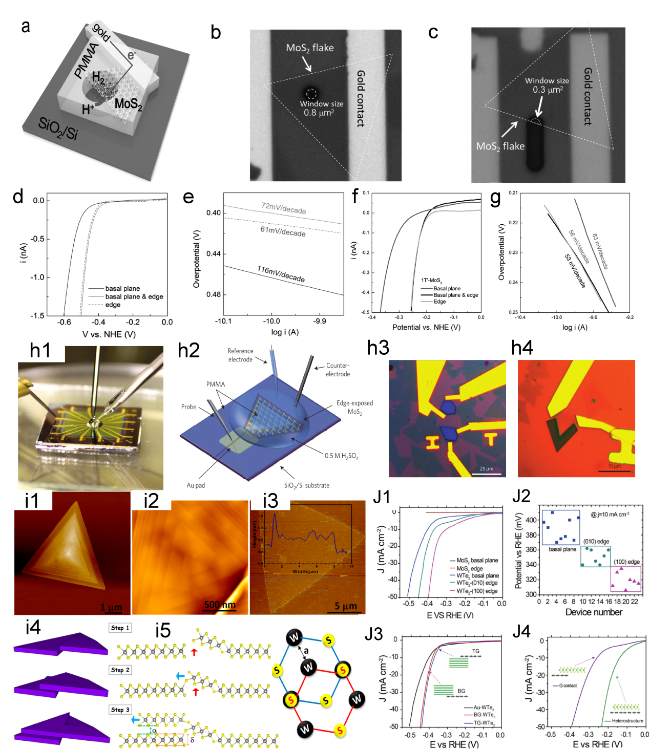
been demonstrated using on-chip microcells.105 106 (Figure 4j1-j4)
Figure 4. (a) Schematic of the on-chip setup. Optical image of a MoS2 showing the (b) basal and (c) edge planes. (d) LSV and (e) Tafel plots of the MoS2 with basal plane and edge, the electrolyte was argon-purged in 0.5 M H2SO4 and scan rate was set at 10 mV s−1. (f) LSV and (g) Tafel curves of monolayer 1T′-MoS2 basal plane, and edge in HER.[64] Copyright 2017, Wiley-VCH Verlag GmbH & Co. (h1) Photograph of the electrochemical microcell. (h2) Schematic of the on-chip electrocatalytic. Optical microscope images of single-layer MoS2 with (h3) the basal and (h4) edge exposed.[80] Copyright 2016, Nature Publishing Group. (i1, i2) AFM of spiral WS2 domains and (i3) corresponding height of monolayer WS2. (i4) Schematic of the spiral domain growth modes. (i5) Schematic of the atomic arrangement in spiral WS2.[104] Copyright 2019, American Chemical Society. (j1) LSV of MoS2 and WTe2 with different exposed area, the overpotential of (100), (010) edge and basal plane is 320 ± 10 mV, 350 ± 10 mV and 390 ± 20 mV respectively at 10 mA cm−2. (j2) Summary of the overpotentials for WTe2 with different exposed sites. (j3) LSV of WTe2 with basal plane. (j4) LSV of MoS2 with a graphene contact, as well as from the MoS2/graphene heterostructure.[105] Copyright 2018, Wiley-VCH Verlag GmbH & Co. |
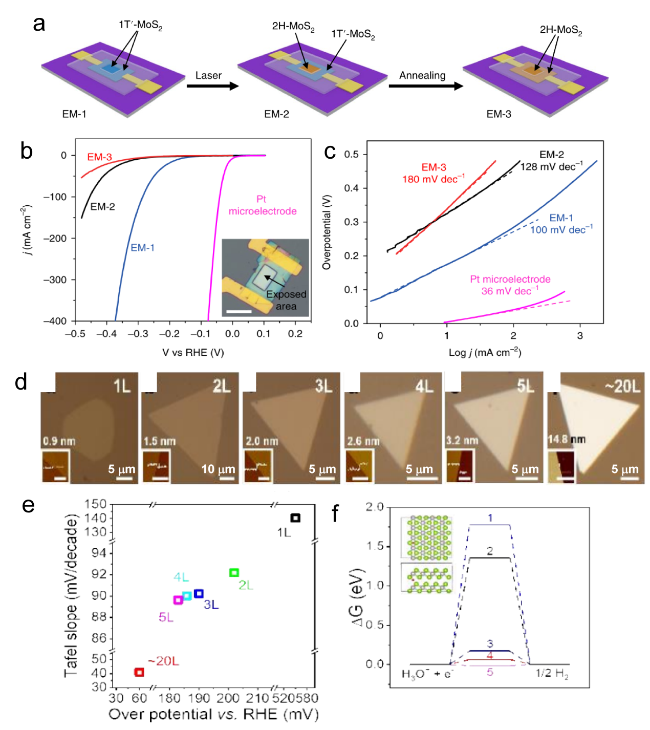
Figure 5. The diagram of fabrication process. (b) The LSV for EM-1, EM-2, and EM-3, with an inset image EM-1, the overpotential of EM-1 and EM-2 is 165 mV and 200 mV, respectively. Scale bar, 20 μm (c) Tafel plots corresponding the (b).[82] Copyright 2018, Nature Publishing Group. (d) Optical images of CVT-grown PtSe2 flakes ranging from 1 to 20 layers, insets show the corresponding AFM images. (the same height scale for AFM images, the scale bar of first is 1 μm, others is 0.5 μm) (e) LSV and Tafel slops for the HER. (f) ΔGH diagram for HER of different layer PtSe2.[106] Copyright 2019, Wiley-VCH Verlag GmbH & Co. |
3.1.2 Methodology for enhancing HER performance
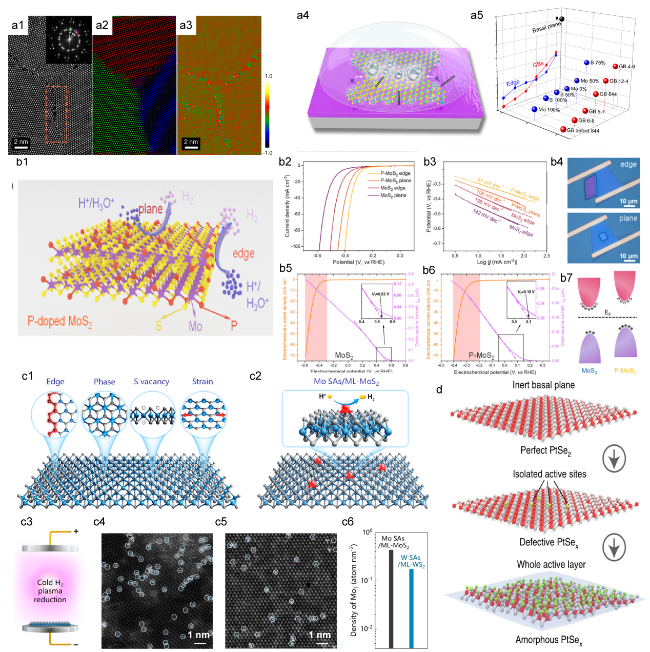
Figure 6. (a1) The HAADF STEM image of the GBs between three MoS2 grains, the inset on the left shows the Fourier transform. (a2) The composite color-coded inverse fast Fourier transform (IFFT) image. (a3) illustrates the Geometrical Phase Analysis (GPA) routine.[112] (b1) Schematic of HER process on P-MoS2 nanosheets. (b2) and (b3) show the LSV and Tafel plots for the MoS2 and P-MoS2, The edge sites show an onset potential (η10) of 297 mV and a Tafel slope of 97 mV dec−1 for HER, exceeding those of the basal plane with 328 mV and 108 mV dec−1. (b4) Optical images of edge and basal plane on a P-MoS2. (b5) and (b6) display the electrochemical and electronic signals of a MoS2 and P-MoS2 during the HER. (b7) Illustrates the electronic structure of P-MoS2, aligning the Fermi levels.[113] Copyright 2017, American Chemical Society. (c1) Schematic of the active sites of MoS2. (c2) Schematic of Mo ML-MoS2. (c3) Details the synthesis of metal SAs on a ML-MoS2. HAADF-STEM images in panels (c4) and (c5) depict Mo SAs on ML-MoS2 and tungsten SAs on monolayer WS2 samples, respectively. (c6) statistical results on the density of Mo and W SAs on 2D monolayers.[114] Copyright 2020, American Chemical Society. (d) Schematic of the amorphous PtSex surface, perfect PtSe2 and defective PtSex.[115] Copyright 2022, Nature Publishing Group. |
rapid hydrogen adsorption and desorption kinetics, thereby enhancing HER activity.[114] To achieve optimal electrocatalytic activity and stability, emerging trends in catalysis are increasingly focusing on the implementation of single-atom noble metal catalysts arranged in a monolayer configuration. This approach allows for the reconfiguration of nearly all atoms to enhance performance. He et al. have recently presented their findings on a wafer-sized amorphous PtSex film, produced through the ion etching of low-temperature argon plasma. Their investigation utilizing a micro-electrochemical cell demonstrated that this amorphous PtSex film, characterized by high atom utilization and a robust single-layer structure, exhibits exceptional catalytic performance that is comparable to that of commercial Pt. [115]
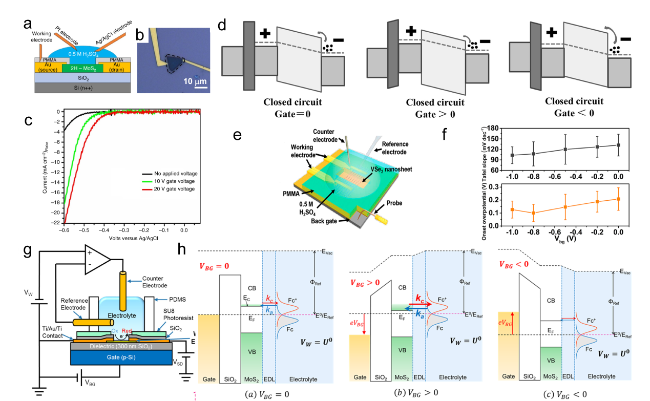
(Figure 7a-c).[119]
Figure 7. (a) Schematic of the gate-modulation electrochemical device. (b) Optical images of 2H-MoS2 with Au pads. (c) LSV of gate-dependent HER measurements, the green and red curves show the improvement in electrocatalytic activity after applying a positive gate voltage of 10 and 20 V, respectively.[119] (d) Energy band diagrams of the MoS2 device at different gate bias.[120] Copyright 2017, Wiley-VCH Verlag GmbH & Co. (e) Schematic of the HER device under back gate voltages. (f) Statistic-based influence of the back gate on the onset overpotential and Tafel slope.[81] Copyright 2017, American Chemical Society. (g) Schematic of a back-gated electrochemical cell. (h) Energy band diagrams of the MoS2 at various back-gate biases.[67] Copyright 2017, American Chemical Society. |
3.2 In situ measurement on-chip device
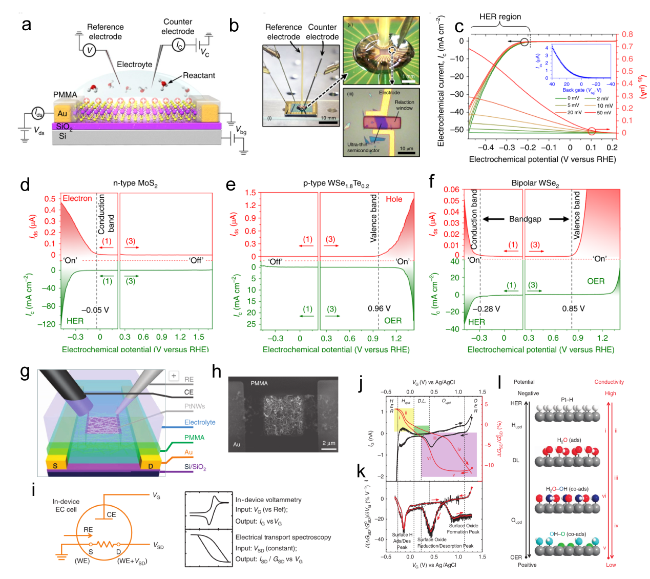
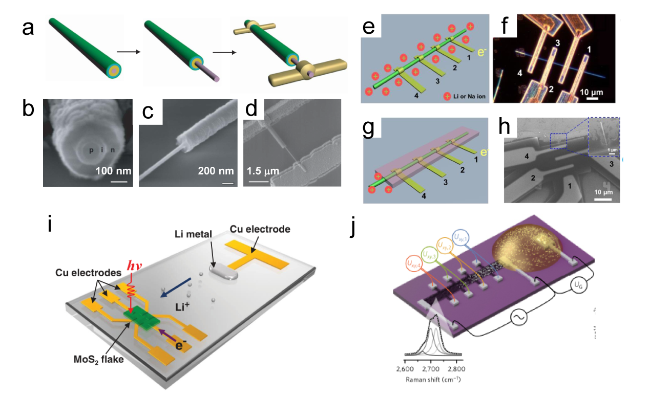
Figure 8. (a) Schematic of the microcell-based in situ electronic/electrochemical measurement. (b) Optical image of the microcell. (c) Typical electrochemical (y axis in black) and electronic (y axis in red) signals of single-layer WS2 during the HER at different bias potentials. Self-gating phenomenon of (d) n-type MoS2, (e) p-type WSe1.8Te0.2, and (f) bipolar WSe2, typically, n-type MoS2 is turned on at a negative electrochemical potential and only delivers the HER, p-type WSe1.8Te0.2 is turned on at a positive electrochemical potential and only delivers the OER, bipolar WSe2 is turned on at both negative and positive electrochemical.[69] Copyright 2019, Nature Publishing Group. (g) Schematic of the Pt NW device. (h) SEM image of the device cell. (i) Schematic of CV and ETS for in situ monitoring of the electrochemical interfaces. (j) The IG-VG and normalized GSD-VG characteristics of a typical Pt NW device, IG-VG resembles the typical CV characteristic of a polycrystalline Pt surface, containing redox regions of HER, H adsorption/desorption region (Hupd), double layer (DL) region, surface oxide formation/reduction region (Oupd) and OER. (k) The differentiated ETS curve illustrates spectral peak characteristics. (l) Schematic of various Pt surface conditions along with the sweeping electrochemical potentials (left black axis) and the corresponding changes in conductivity.[68] |
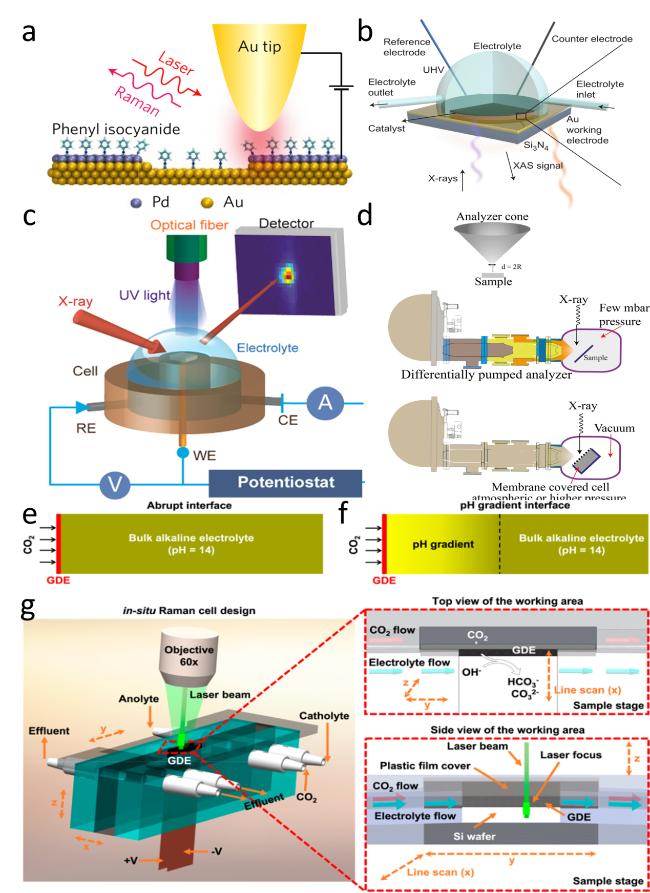
Figure 9. (a) Schematic of an STM-based time-dependent tip-enhanced Raman spectroscopy (TERS).[140] Copyright 2016, Nature Publishing Group. (b) Schematic illustration of the in situ XAS liquid cell.[142] Copyright 2018, Nature Publishing Group. (c) Schematic of the experimental setup for operando X-ray reflectivity of SrTiO3.[141] Copyright 2016, American Chemical Society. (d) Schematic illustration of the in situ XPS experimental set-up.[143] Copyright 2018, Wiley-VCH Verlag GmbH & Co. Designed flow cell for performing in situ Raman measurements to distinguish between (e) an abrupt interface and (f) a gradient interface. (g) The cell design with both top and side views of the cathode area.[144] Copyright 2020, American Chemical Society. |
Table 1 Summary of on-chip HER electrocatalytic devices. |
| Strategies | Types | Materials | Performances | Electrolyte | Ref. |
|---|---|---|---|---|---|
| Identification of active sites | Basal and edge | 2H and 1T'-MoS2 monolayers | (2H)basalη10=−425±27 mV Edge η10=−201±42 mV (1T')basalη10=−356±41 mV Edge η10= −77 ± 24 mV | 0.5 M H2SO4 | [64] |
| 2H-MoS2 basal plane with the vacancy | η10 ≤ −150 mV | 0.5 M H2SO4 | [80] | ||
| helical WS2 | η=−560 mV (vs Ag/AgCl) (20 nA μm−2) | 0.5 M H2SO4 | [104] | ||
| WTe2 | η(100)= −320 mV | 0.5 M H2SO4 | [105] | ||
| phase and layer | 1T'-MoS2 2H/1T′-MoS2 | η= −65 mV η= 200 mV | 0.5 M H2SO4 | [82] | |
| Heterophase boundaries between the 2H and 1T’ phases in MoTe2 | η= -210 mV | 0.5 M H2SO4 | [107] | ||
| PtSe2 | Monolayer η=60 mV Thick η=550 mV | 0.5 M H2SO4 | [106] | ||
| Catalytic window | 2H-MoS2 | η=−290 mV | 0.5 M H2SO4 | [108] | |
| Monitoring the performance at a single material. | GBs and S vacancies | MoS2 nanograin film | η10=−25 mV | 0.5 M H2SO4 | [112] |
| Doping | P-MoS2 | η10=−297 mV | 0.5 M H2SO4 | [113] | |
| V-MoS2 | η10=−185 mV | 0.5 M H2SO4 | [116] | ||
| Design single atom | Mo-MoS2 | η10=−107 mV | 0.5 M H2SO4 | [145] | |
| Amorphous PtSex | η=−100 mV | 0.5 M H2SO4 | [115] | ||
| Construct Heterostructure | MoS2/graphene | η10=−110 mV | 0.5 M H2SO4 | [105] | |
| Methylene blue (MB)/MoS2 interfaces | η10=−206 mV | 0.5 mM MB | [118] | ||
| Electric Field Modulation | MoOx/MoS2 core-shell nanowires | η=−200 mV | 0.5 M H2SO4 | [119] | |
| MoS2 at the gate voltage of 5 V | η10=−38 mV | 0.5 M H2SO4 | [120] | ||
| VSe2 | η10=−126 mV | 0.5 M H2SO4 | [81] | ||
| WSe2 with back-gate voltage 20 V | η10=−280 mV | 0.5 M H2SO4 | [124] | ||
| (CoPc)/MoS2 with back-gate voltage 2V | η10=−238 mV | 0.5 M H2SO4 | [130] | ||
| Pt SAs on n-type MoS2 with Vg +40 V | η10=−20 mV | 0.5 M H2SO4 | [130] | ||
| Thermal Modulation | inert MoS2 ML basal plane at 60 °C | η10=−90mV | 0.5 M H2SO4 | [131] |
3.3 Oxygen evolution reaction (OER) on-chip device
3.4 Water splitting on-chip device
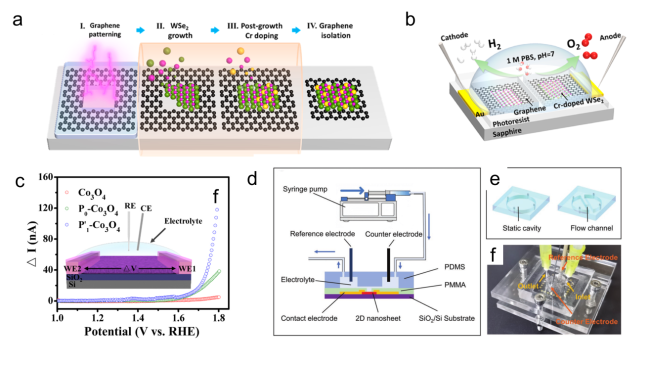
Figure 10. (a) Schematic of the formation process of the Cr-WSe2/graphene heterojunction. (b) Schematic of the on-chip electrocatalytic device.[85] Copyright 2022, American Chemical Society. (c) In situ I-V measurements of individual Co3O4, P0-Co3O4 and P-Co3O4 thin-films. Inset: The schematic of in situ I-V measurements. The applied potentials in WE1 and WE2 (called V1 and V2) increase synchronously vs. RHE, from 1 to 1.8 V, and keep the constant tiny potential difference ΔV (ΔV=1 mV).[159] Copyright 2021, Elsevier Ltd. All rights reserved. (d) On-chip electrochemical measurement setup for the ORR. (e) Schematic illustration of the setup. (f) Schematic diagram of the static and microfluidic PDMS cells.[84] Copyright 2022, Wiley-VCH Verlag GmbH & Co. |
3.5 Oxygen reduction reaction (ORR) micro-device
3.6 CO2 reduction reaction (CO2RR) and Nitrogen reduction reaction (NRR) on-chip device
3.7 Bio-electrochemical on-chip device
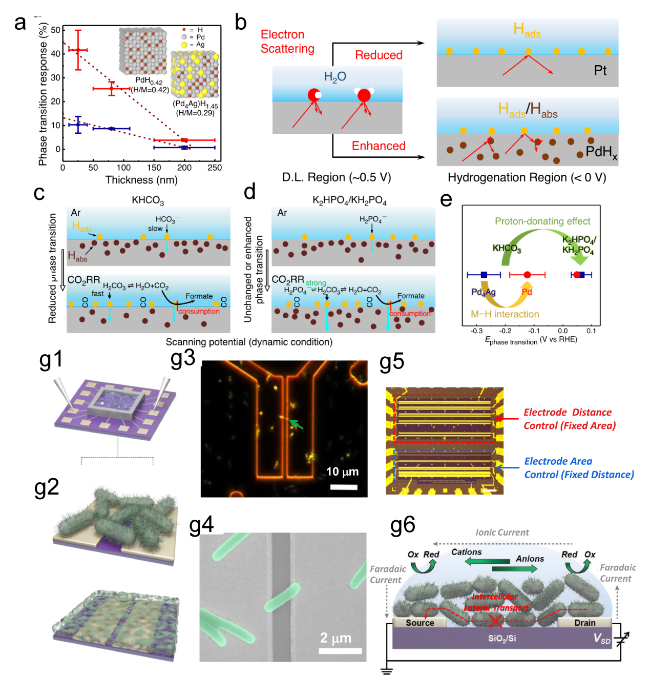
Figure 11. (a) The relationship between the thicknesses of Pd and Pd4Ag nanowire films and their phase transition responses (ΔRMHx) is illustrated on the ETS. (b) Schematic of electron scattering in metals with surface adsorbates and hydrides. A schematic of the different Pd-H states in (c) KHCO3 and (d) K2HPO4/KH2PO4 and the corresponding CO2RR processes at the interfaces. (e) Summary of phase transition potentials of Pd and Pd4Ag under CO2RR conditions in KHCO3 and K2HPO4/KH2PO4 obtained at 10 mV/s.[172] (g1, g2) Schematic of the nanoelectronic measurement setup. (g3) Optical microscope (OM) image of MR-1 in dark-field mode. (g4) An ex situ SEM image of MR-1. (g5) Schematic of biofilm measurements. (g6) Schematic of the electrochemistry model at the interface of Shewanella oneidensis MR-1.[173] Copyright 2016, American Chemical Society. |
3.8 Photoelectrochemical on-chip device
Figure 12. (a) Schematics of fabrication process of the single nanowire photovoltaic device. (b, c, d) SEM images corresponding to schematics in a.[91] Copyright 2007, Nature Publishing Group. (e, f) The schematics and dark field optical microscopic image of the nanowire electrode. (g) The second configuration, in which the nanowire is covered by a passivation layer with only one exposed end. (h) The SEM image of the device with the second configuration.[79] Copyright 2015, American Chemical Society. (i) Schematic of MoS2-Li microbattery.[180] Copyright 2015, Wiley-VCH Verlag GmbH & Co. (j) Schematic of the device for lithium ion diffusion in bilayer graphene. The inset shows the Raman scattering response of bilayer graphene.[181] Copyright 2017, Nature Publishing Group. |
3.9 Mechanism analysis
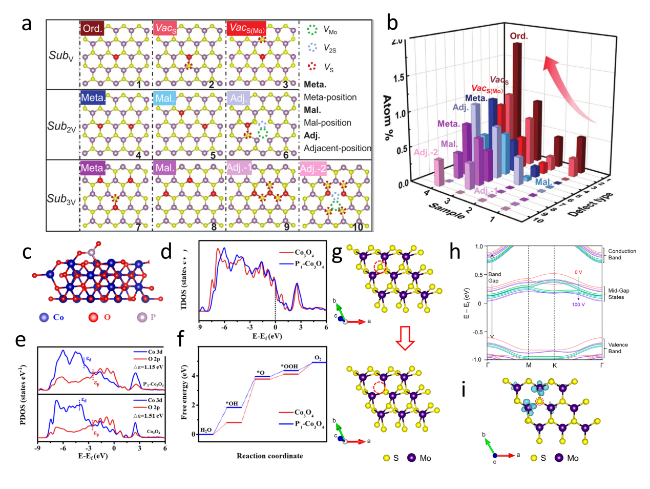
Figure 13. (a) Illustration of atomic defects observed in single-layer V-MoS2. (b) Statistical analysis of the concentration of these atomic defects.[116] Copyright 2022, Wiley-VCH Verlag GmbH & Co. (c) Structural diagram of P-Co3O4. (d) Total electronic density of states for Co3O4 and P-Co3O4. (e) Calculated PDOS of Co3O4 and P-Co3O4. (f) Calculated free energy diagram of the OER on Co3O4 and P- Co3O4.[159] Copyright 2021, Elsevier Ltd. All rights reserved. (g) The schematic of the S vacancy site created on the modeled MoS2 surface. (h) The computed band structures of the MoS2 surface with 5.5% S vacancies at various induced charge levels. (i) The charge density difference of a monolayer of MoS2 with 5.5% S vacancies.[192] Copyright 2019, American Chemical Society. |
4 Energy storage devices
4.1 Battery on-chip device
4.2 Micro-supercapacitors on-chip device
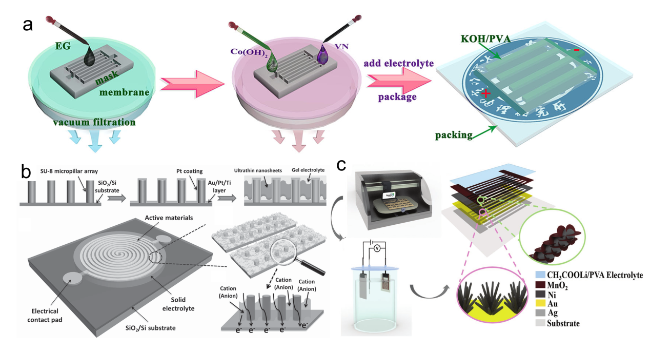
Figure 14. (a) Fabrication and structural characterization of SST-MPCs.[205] Copyright 2018, Nature Publishing Group. (b) Schematic of the fabrication process of SST-MPCs.[206] Copyright 2015, Wiley-VCH Verlag GmbH & Co. (c) Schematic of the printable fabrication procedure.[207] Copyright 2017, Wiley-VCH Verlag GmbH & Co. |
5. Conclusion and perspective
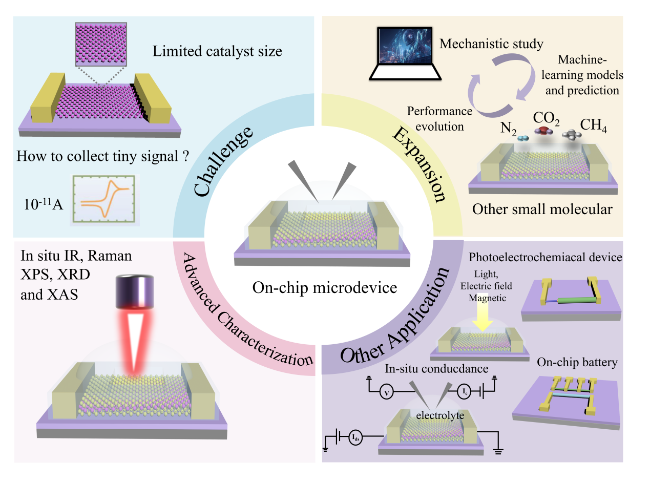
Figure 15. Prospects for on-chip microcells. The challenges in this emergent field, such as limited catalyst size. The applications of the on-chip microcells, e.g., the expansion, other application and advanced characterization are expected. |